Omega-3 Supplementation For Liver Health
Doesn’t fat make you… fat? Happy answer, not if you consume the right fats.
From the author: I originally wrote this piece last year when I was just fresh in the nutrition program, and I was very passionate about this subject. Fast forward over a year, I now have finished the program and have been fortunate to be working on an omega-3 and fatty liver related project. I’m very happy to report that after digging through a more than comprehensive list of primary research papers, this is the conclusion I came to: omega-3 supplementation does yield beneficial outcomes for individuals with non-alcoholic fatty liver disease. My apologies for this piece being a heavier read with a lot of technical information, for it started off as a passion project.
I hope you enjoy the read.

“Enriched with omega-3” seems to be the catchphrase nowadays for any fat-containing products. In recent years, omega-3 fatty acids are gaining tremendous attention. While the media coverage of omega-3 supplementations is widely positive, there is still a certain degree of skepticism to what this magical fat actually does to the human body. A rather intuitive question I was confronted with by a family member was “does omega-3 make you have a fatty liver?” The happy answer to this question, however, is a very likely “no” supported by scientific evidence. Furthermore, not only would omega-3 not contribute to the development of fatty liver, it might actually help reduce liver fat accumulation. In this mini-review, I’m breaking down the topic into 5 parts: 1) what omega-3 fatty acids are; 2) what causes the development of a fatty liver; and 3) what is the scientific evidence that omega-3 is beneficial (for preventing/reducing fatty liver) and how it works, 4) results from human clinical trials; and 5) things to consider when taking omega-3. Feel free to hop to the section you’re most interested in!
What are omega-3 fatty acids?
To understand how omega-3 works in the body, it is important to understand what it is. These are the essential fats for the human body as we cannot produce them ourselves. Chemically, omega-3 is a group of fatty acids that has a double bond at the third carbon position in the carbon chain, making the chain not saturated with hydrogen bonds (like in the chemical structure of DHA). Due to the numerous double bonds in the carbon chain, omega-3 fatty acids are also named omega-3 polyunsaturated fatty acids (PUFAs) [5,21].
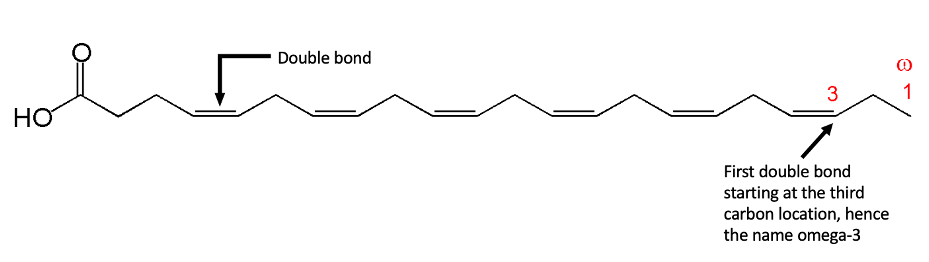
The double bonds in PUFAs allow them to twist and fold into non-uniform shapes, so they don’t stack up and cause clogs in the body [5].
Omega-3 PUFAs also come in different shapes and forms. Typically, the very-long-carbon-chain (>20 carbons) variation of the omega-3 PUFAs yields the most benefits, like DHA and EPA you’ll find in a fish oil product. A shorter form of dietary omega-3 PUFA is the 18-carbon ALA (alpha-linolenic acid), which is found rich in seeds and nuts (ex. chia and flax seeds) [5,21]. Here, we’re mainly focusing on EPA and DHA, the two types most commonly found in fish oil.
Fatty liver diseases: development and symptoms
Intuitively, one might wonder “doesn’t fat make you….. fat?” Well, the answer is not so black and white, as the cells in our body only really store fat when there is a surplus of energy. To understand this, we should really look at fats for one of their primary biological purposes: to store energy. With this viewpoint, it is easy to see that excess energy needs to be burnt off to rid the excess fat. A popular measuring unit for biological energy is calories. Since each gram of fat yields 9 calories, compared to 4 calories from 1 gram of either carbohydrates or protein, eating too much fat does have a tendency to introduce too much energy [5]. This leads to fat buildup. However, if eaten moderately, the ingestion of fat does not inherently make someone fat (ie. the keto-diet, but I’ll refrain from commenting on it).
This then leads to the second part of this topic on the regulation of fat deposition in the liver.
A fatty liver is defined by a >5% fat content of the total liver mass. The presence of a fatty liver can be due to many causes, but the core idea is an unbalance between fatty acids in and out of the liver [16].
Ways of fats into the liver:
- dietary fats absorbed by the intestines, delivered to the liver in the form of lipid droplets called chylomicrons via bloodstreams
- fatty acids broken down from fat cells in the body via bloodstreams
- synthesis of fat molecules by the liver cells via de novo lipogenesis
Ways of fats out of the liver:
- generation of ketone-bodies by breaking the fatty acids down via oxidation
- make fat molecules from circulating free fatty acids, and transporting the fats in the form of another lipid droplet called very-low-density-lipoprotein (VLDL) to the rest of the body (for excretion or storage)
An ideal situation would be when fats IN and OUT of the liver are balanced. The problem is, VLDL secretion reaches a plateau at a certain level as shown in research [4]. In addition, high sugar intake results in high blood insulin level; insulin activates enzymes that make and store fats [13]. Lastly, oxidation of fatty acids in the liver is regulated by PPAR-α, a key regulatory protein in fat metabolism in the liver, and whose activity is enhanced during starvation state [8].
In simple terms, the development of a fatty liver is an outcome of a combination including:
- insufficient export of the fats from the liver
- a slow oxidation process
- a high rate of fat-synthesis (lipogenesis) due to metabolic diseases or excess energy intake
Omega-3 and fatty liver diseases
So how does the supplementation of omega-3 PUFAs tie into the lipid metabolism in the liver? In the late 2000s and early 2010s, scientists had come to the consensus that omega-3 supplementation can help reduce fat accumulation in the liver cells by regulating the expression of genes involved in both sides of the “fat in vs. out” equation.
First off, omega-3 PUFAs can reduce the amount of fat introduced to the liver by inhibiting the production of fat (lipogenesis). In fact, long-chain omega-3 PUFAs (EPA and DHA) were shown to reduce the production of mature SREBP-1 protein (a key-player in fat production) by aiding the break down of the immature intermediates, which essentially leads to the reduction of fatty acids synthesized by the liver [18]. The less free fatty acids floating around in the liver, the less fat accumulation there is within the liver cells.
On the other hand, omega-3 PUFAs can increase the rate of “fat out” in the liver. Specifically, omega-3 PUFAs had also been shown to increase the break down of liver fat by increasing the production of PPAR-α [25], which was mentioned earlier as a key player in the fat oxidation process.
Moreover, omega-3 supplementation may also reduce insulin resistance systematically, thus increasing energy burning from both fat and carbohydrates. A very interesting cell culture study in 2015 used liver cells as a model and grew the cells in media rich in different types of fats, including omega-3 PUFAs. Afterwards, they had soaked the cells in a solution rich in free fatty acids to mimic a fatty liver environment, in hopes to induce cell lipotoxicity. What they had found was the protective effects of omega-3 PUFAs on the liver cells against cell death, upon induction of cell damage by free fatty acids [3]. In identifying insulin resistance, the accumulation of lipids in non-fat tissues leading to lipotoxicity is one main factor for the development of insulin resistance. A high fat environment thus is considered deleterious. On the other hand, mitochondrial dysfunction as a result of overnutrition also plays a significant role in insulin resistance, together with oxidative stress on the cell, causing inflammation. Omega-3 PUFAs had shown to have anti-inflammation properties and thus can aid in regulation of proper cellular metabolism [27]. This is especially important in an already fatty environment as in the case of fatty liver disease.
Omega-3 in human clinical trials
One of the challenges scientific researches face is the translation of drug effects from cell and animal experiments into human clinical trials. In this case, omega-3 supplementation is of no exception. So far, many randomized human trials had been conducted on omega-3 PUFAs as a treatment for non-alcoholic fatty liver disease (NAFLD), in both adults and children patients. Though still a mixed pool of results, most studies did report benefits of the daily n-3 consumption, including the following:
- improved lipid metabolism, shown in reduced blood lipid level [6,10]
- reduced liver damage, shown in reduced blood ALT and AST (liver enzymes) level [6,22]
- improved liver steatosis, or a lower NAFLD Activity Score at the end of trials [22,23,24]
- improved whole body and liver insulin sensitivity [10,23]
The dosages of omega-3 supplementations used in these studies range from 1000mg/day to 4000mg/day, with a general trend of high-dose omega-3 yielding more significant results. These studies also used a variety of n-3 PUFA forms, including purified DHA, EPA, a purified mixture of both, fish oil, seal oil, or algae oil.
On the other hand, some studies had reported insignificant findings with regard to the effects of n-3 supplementation. One recent randomized trial (2019) compared the outcomes from a calorie-restriction diet (350-700kcal daily deficit) vs. omega-3 (EPA and DHA) supplementation with no dietary interventions, in patients with BMI 25-40 (overweight/obesity) and liver steatosis. The study showed significant weight loss, improved AST level and insulin sensitivity, and reduced blood lipid levels, in patients receiving the dietary intervention, but not in the n-3 group [15]. Although I personally don’t think n-3 supplementation and a dietary restriction are necessarily comparable, it does seem as though the omega-3 group outcome didn’t differ significantly from the dietary intervention group’s. Another study using EPA supplementation also noted “no significant effect on histologic features of [non-alcoholic steatohepatitis]” with 1.8g/day or 2.7g/day of EPA ethyl ester. The study did report an unexpected higher patient dropout rate compared to the original design, so the lack of power might contribute to the lack of therapeutic response [14]. It is also worth noting that only some studies tracked the change in cellular omega-3 PUFA levels, an indicator of omega-3 uptake into the human body. Some studies had found the placebo group also had elevated cellular PUFA level, a sign of off-protocol omega-3 intake [24]. Therefore, the diets of participants prior and during each trial should be closely monitored and adjusted for.
One interesting observation I found though is the variety of different forms omega-3 supplementations can take and how they’re differently sourced. This may be an interesting variable to test in the future, with some examples of omega-3 supplements taking forms of seal oil, fish oil, algae oil, purified ethyl esters, and many others.
Omega-3 supplementation safety concerns
Perhaps the biggest concern with omega-3 supplementation in patients so far had been bleeding in high-dose supplementations. There had been some limited clinical evidence showing that omega-3 PUFAs can interact with Warfarin (anticoagulants) and increase bleeding in patients [2,7]. The NIH of the United States also recommends patients to discuss n-3 supplementations with their healthcare providers [20]. However, numerous other studies had found doses of 3-6g of daily fish oil consumption to not disturb blood clotting [11, 17,19]. A large randomized trial published in 2018 on fish oil consumption (1-2g/day) in patients going under cardiac surgeries also found fish oil to be safe [26].
One strictly controlled randomized study noted the potential adverse effect of n-3 PUFA supplementation in patients with type II diabetes (DM), as the NAFLD Activity Score changes in the PUFA group was less than in the placebo group. This study related this to the potential deleterious effects of induced cellular oxidative stress by activation of PPARα, which as mentioned earlier is a regulator in lipid metabolism [25]. This result is directly contradictory to findings in other studies, and is concerning if true. A few studies did also observe increased blood sugar levels in T2D patients consuming n-3 PUFAs, which is another safety concern [28]. Although, one study found aerobic exercise abolishes this deleterious effect [28]. Personally, I haven’t seen many government official websites mentioning the potential safety concern of omega-3 consumption in DM patients, and I would love to see follow up experiments on this.
As noted by the NIH, omega-3 PUFA consumption may lead to abdominal discomforts, but a dosage of <5g/day should be safe for daily consumption [20].

The bottom line
The bottom line? Long-chain omega-3 PUFA supplementation can yield beneficial outcomes in the case of fatty liver disease, as seen in many human trials. A few meta-analyses and review papers on omega-3 supplementation and liver health had summarized the evidence for omega-3 PUFAs’ beneficial effects in both children and adults with nonalcoholic fatty liver disease (NAFLD) [1,9,12]. This is not to say that omega-3 is a cure-all, and one should certainly not indulge in 10 grams of omega-3 per day. An effective and safe dosage recommended by Health Canada is 1-5 grams per day. Taken together, it is safe to conclude that it would be the most beneficial for any conscious consumers to replace some of the daily fats with omega-3, while maintaining the same caloric intake.
***Disclaimer: we’re not affiliated with any organizations for the writing of this article. This article is not medical advice; please consult your healthcare provider for further information on omega-3 consumption for your individual situation.